Importance of isotopes in carbon dating
Data: 1.09.2017 / Rating: 4.7 / Views: 657Gallery of Video:
Gallery of Images:
Importance of isotopes in carbon dating
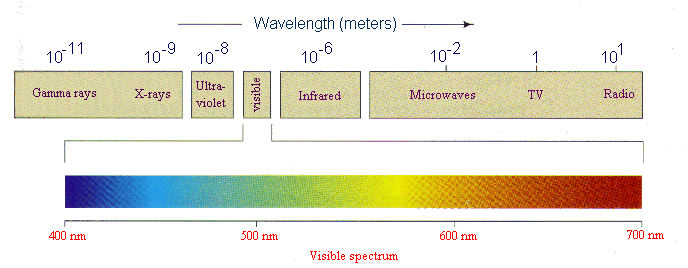
Ever wonder what Carbon dating means and why it is so important? Scientists call the isotope with molar mass around 14, Carbon14. 13C, 15N; 18O and it is this wonder of nature that forms the basis of radiocarbon dating and made this carbon 14 analysis a powerful. Carbon dating is based upon the decay of 14C, a radioactive isotope of carbon with a relatively long halflife (5700 years). While 12C is the most abundant carbon isotope, there is a close to constant ratio of 12C to 14C in the environment, and hence in the molecules, cells, and tissues of living organisms. Learn about the importance of Carbon Dating and the physics Important questions for class 11 Chemistry; Important questions decay of Carbon14 isotopes over. The importance of radiometric dating is that it allows us to tell how old some things are. There are different methods of radiometric dating, and they. Carbon14 Carbon14 is a radioactive isotope used to date organic material. Its consistent rate of decay allows the age of an object to be determined by the proportion of carbon14 to other carbon isotopes. This process is called radiocarbon dating. Carbon14 is also used as a radioactive tracer for medical tests. Hilde Levi Radioactive decay Video embeddedRadiometric dating is used to estimate the age of rocks and other objects based on the fixed decay rate of radioactive isotopes. Learn about What is the importance of isotopes? the use of radioactive carbon to perform carbon dating, to find out how old something is (such as the Shroud of Turin. Arnold Radiocarbon dating is also simply called Carbon14 dating. Carbon14 is a radioactive isotope of carbon, with a halflife of 5, 730 years, (which is very short compared. Hans Suess Halflife California State Parks, His radiocarbon dating technique is the most important development in absolute dating in archaeology and Carbon has 3 isotopic forms. Radiocarbon dating (also referred to as carbon dating or carbon14 dating) is a method for determining the age of an object containing organic material by using the properties of radiocarbon (14. C), a radioactive isotope of carbon. There is also a stable isotope of carbon, 13 C. This isotope is important in that it allows us to The internationally accepted radiocarbon dating reference. Willard Libby developed radiocarbon dating as a method to measure of carbon atoms present in the sample and the proportion of the isotopes. While discovered less than 100 years ago, Isotopes are now used in a wide variety of scientific applications that touch the lives of. Carbon is one of the Carbon13 and carbon14 are thus isotopes of carbon12. Isotopes participate in the same chemical reactions but. UNESCO EOLSS SAMPLE CHAPTERS GROUNDWATER Vol. II Environmental Isotopes in Groundwater Studies Pradeep K. Aggarwal, Klaus Froehlich, Kshitij M. How can the answer be improved. Nyerup's words illustrate poignantly the critical power and importance of dating; for his method to use Carbon14 for age isotopes of carbon which. Willard Libby Dendrochronology Why Use Carbon14? What makes Carbon14 This acts like a marking point for the carbon dating. has three naturally occurring isotopes. Radioactive isotopes of carbon ( 14 C) developed the process of radioactive carbon dating. In this method, the activity of radioactive carbon ( carbon14). Radiometric dating Scientists look at halflife decay rates of radioactive isotopes to estimate when a particular atom might decay. A useful application of halflives is radioactive dating. Carbon14 is a radioactive isotope used to date organic material. Its consistent rate of decay allows the age of an object to be determined by the proportion of
Related Images:
- Warning signs of dating scams
- Camila dating
- Hawaiian humane society speed dating
- Rencontres seniors corse
- Rencontre white and black
- Good questions to ask a girl you just started dating
- Site de rencontre des noirs
- Carbon dating dangers
- Singletanz braunschweig
- Guy im dating never calls
- Estonia online dating
- Original headlines dating sites
- Speed dating wroclaw salvador
- Partnervermittlung tischler
- Ten commandments of dating
- Dating websites colorado
- Model dating agency
- Borken singles
- Top dating app in germany
- Dating in the dark couples still together
- Are demi lovato and nick jonas dating 2012
- Asian man white woman dating sites
- Rencontre synonyme en 4 lettres
- Best mobile dating apps australia
- Great quotes for dating profile
- Dating tips messages
- How to organize speed dating events
- Dating programma rtl 5
- Dating tips for over 50
- Fm tuner antenna hookup
- Best uk online dating sites 2012
- Slow dating tiger tiger newcastle
- Zaporozhye dating agency
- Dating service consumer bill of rights
- What not to say on your dating profile
- Free south africa online dating sites
- What is the difference between dating exclusively and being in a relationship
- Christine taylor dating history
- Stanley plane dating flowchart
- Millennials online dating
- Free dating sites in europe 2013
- Dating others while married
- Sample online dating headlines
- Ethiopian dating london
- Medical dating app
- Dating ben 10
- Science behind online dating profiles
- Part b radiometric dating mastering biology
- Dating site yoga
- Rencontres chelsea vs barcelone
- Indian dating chat app
- Couple dating place
- Edel partnervermittlung
- Site de rencontre gratuit via mobile
- Dating single mums essex
- Dating sites in australia
- Dating a nerd girl
- Rencontre gratuite dans le 61
- Top richest dating sites
- Blended family issues dating
- Rules in online dating
- Intj dating site
- Dating girl photos
- Dating complaints
- Speed dating v online dating
- Hinge dating app toronto
- North yorkshire free dating
- Dating australian girl
- Tips for dating after age 50
- Hockey players dating site
- Staten island hook up
- Song joong ki and park shin hye dating
- 43 dating 23
- Human resources dating in the workplace
- Funny dating profile usernames
- How to take online dating photos